Nestlé has issued a voluntary recall for specific batches of its SMA infant and follow-on formula products in Ireland due to the potential presence of a toxin that can cause food poisoning, the Food Safety Authority of Ireland (FSAI) said on Monday.
The food safety regulator warned that the products may contain cereulide, a toxin produced by certain strains of the bacterium Bacillus cereus. While no illnesses have been reported to date, the FSAI described the recall as a “precautionary measure” taken in the interest of public health.
The FSAI advised parents, guardians, and caregivers to immediately stop using the affected batches. Consumption of foods containing the cereulide toxin can lead to nausea and severe vomiting, with symptoms typically appearing within five hours of ingestion. The resulting illness generally lasts between 6 and 24 hours.
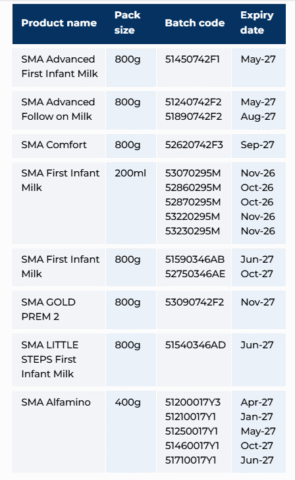
Officials noted that the toxin is extremely heat resistant and can be pre-formed in food products. The recall affects both powdered formulas and ready-to-feed containers. Batch codes for the powdered products are located on the base of the tin or box, while codes for liquid formulas can be found on the side or top of the container and the base of the outer packaging.
The regulator stated that if a child has consumed the product but shows no symptoms, no further action is required. However, caregivers are urged to contact a healthcare professional if they have concerns regarding the health of an infant or young child.
Nestlé has instructed customers who purchased the affected batches to contact the company via its online form or through its dedicated careline. Consumers in Ireland can reach the company at 1800 931 832, while those in the United Kingdom can call 0800 0 81 81 80. Recall notices will be displayed at point-of-sale.
In a statement, Nestlé said: “All other Nestlé products and other batches of the same product that are not in the scope of this voluntary recall are safe to consume.
“We understand that this news may cause concern for parents, and we sincerely apologise for any concern or inconvenience caused to parents, caregivers, and customers.”
The affected products are as follows: